 A Trojan horse full of molecular miscreants rolls into an immune cell—the kind that’s supposed to defend the body against this sort of thing. When the timing is just right, the infiltrators burst out, overtaking the cell and forcing it to build more horses and more armies so they can move on and do this again. Which they do, essentially burning the place down on their way out. In the ensuing years, the body’s would-be outposts, known as CD4 T cells, dwindle, giving free rein to whatever other ne’er-do-wells might come along next.
A Trojan horse full of molecular miscreants rolls into an immune cell—the kind that’s supposed to defend the body against this sort of thing. When the timing is just right, the infiltrators burst out, overtaking the cell and forcing it to build more horses and more armies so they can move on and do this again. Which they do, essentially burning the place down on their way out. In the ensuing years, the body’s would-be outposts, known as CD4 T cells, dwindle, giving free rein to whatever other ne’er-do-wells might come along next.
Which they do. In time, the whole empire falls.
The story of the horse is the story of HIV. A retrovirus, it co-opts certain critical white blood cells, putting its own genetic material right into their nuclei. Current treatments target the enzymes that duplicate HIV’s genome and force it into the host cell’s genome. These are the villains within the virion that turn CD4s into HIV’s own munitions plants.
HIV is pretty sloppy about its viral genome duplication processes, but that works out in its favor. Little genetic improvisations churn out new mutants all the time, so this deadly retrovirus—in its perpetual state of reinvention—is a moving target. Drug resistance is a constant threat.
But what if we could stop these dictators before they could even get inside the cell in the first place? What if, instead of trying to take out these individual villains, we could sabotage their Trojan horse, the protective protein coat known as the capsid? It’s a target rich with possibilities because, as with Virgil’s fabled equine, when it comes to opening the capsid, timing is everything.
“The capsid has to remain intact to protect the HIV genome and get into the human cell,” says Peijun Zhang, an associate professor of structural biology at the University of Pittsburgh. “But once inside, it has to come apart to release its contents so that the virus can replicate.”
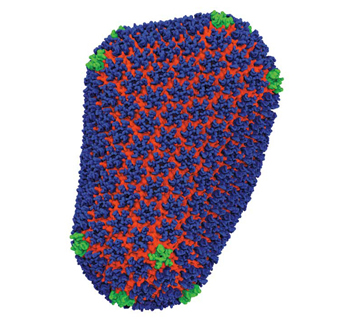
Treatments for many other viruses target capsids. But no one has managed to do this with HIV because, as capsids go, it’s a doozy. While other capsids are uniform, soccer-ball-shaped—predictable—HIV’s is asymmetrical, cone-shaped, and arranged in a confounding lattice of six-pointed and five-pointed structures—the exact numbers and configurations of which were unclear. No two HIV capsids are exactly alike, so the typical approach of structural biologists—to average together corresponding dimensions from many sample structures—wouldn’t fly with HIV.
To make matters worse, the HIV capsid is big. With current imaging tools, you can only get a couple high-resolution images of small sections of the capsid, or low-res images of wide-angle shots.
Then, a few years ago, Zhang decided to apply a hybrid approach, to get the best of both worlds. (With a BA in electrical engineering, an MA in solid state physics, and a PhD in biophysics, Zhang is a bit of a hybrid herself.) In May, her efforts within the multidisciplinary, multi-institutional Pittsburgh Center for HIV Protein Interactions (PCHPI) culminated when the HIV capsid—one of the largest biological molecules ever solved—made the cover of Nature.
It all began with one of those right-place-right-time stories. Zhang, a recruit from the National Cancer Institute, happened to arrive at Pitt in the summer of 2006, just before the National Institutes of Health put out a call for applications for a new HIV center grant—which Pitt’s Angela Gronenborn, a PhD and UPMC-Rosalind Franklin Professor and Chair of the Department of Structural Biology, decided to go for. And which the Pitt team got, opening the PCHPI in 2007. “The NIH only funded three centers, and we were one of them,” says Zhang. “And the other two centers had a long history of studying HIV; we were the new kid on the block.” (Pitt was renewed for this five-year grant in 2012.)
Zhang had never worked on HIV before. But she had been working for more than a decade with cryoelectron microscopy (cryo-EM), a method of freezing specimens at liquid-nitrogen temperatures, then magnifying them through a beam of electrons just a fraction of an angstrom wide. It’s a favorite tool of structural biologists because it allows them to peek at large protein complexes in their native environments—no chemical treatment, no staining, no worries about compromising the integrity of the structures. (For Zhang’s PhD thesis, she solved the structure of a vital calcium pump, involved in muscle contraction, to the 8-angstrom level. This, too, was published in Nature.)
In 2008, Zhang began working with HIV capsid protein. She used purified protein, expressed and prepared by PCHPI investigator Jinwoo Ahn, assembled it into highly ordered helical tubes under high-salt conditions, and imaged them using cryo-EM. Cryo-EM is so sensitive it picks up not only the specimen you’re studying but also an awful lot of visual “noise” around it. To tune that out, you have to average together a lot of images—the more the better. But given the variability among the HIV capsids, she realized she needed to compare apples to apples, so she selected her tubes carefully, including in her analysis only those that shared the same geometrical organization.
About 18 months and more than 34,000 carefully picked apples later, Zhang’s group had an 8-angstrom structural map, which she calls “beautiful hexagonal flower patterns”. The structure revealed critical molecular interactions—previously unrecognized—at the seams of the capsid assembly. PCHPI investigator Christopher Aiken, a professor of pathology, of microbiology, and of immunology at Vanderbilt University, further tested and validated the roles of those interactions in HIV infection.
Map in hand, the PCHPI team decided to push ahead to build a working model of the complete HIV capsid using the structural insights gleaned from the capsid tubes. By converting cryo-EM data on the tubes into high-resolution 3-D images and combining those with results from cryo-electron tomography regarding the shapes of authentic core samples from the Aiken lab, they hoped to calculate the 3-D structures of native authentic HIV-1 cores.
The aspiration would have remained just an untested rough draft if not for another bit of serendipity. Gronenborn called on her colleagues in the Theoretical and Computational Biophysics Group (TCBG) and the NIH Center for Macromolecular Modeling and Bioinformatics at the University of Illinois at Urbana-Champaign (UIUC). Scientists there undertook a heroic effort, investing myriad hours of original work as they applied their own, innovative software (six years in the making) to Zhang’s data. Without their collaboration, says Gronenborn, the project simply wouldn’t have progressed.
Using their “molecular dynamics flexible fitting” software, the Illinois group created a simulation that integrated the known physical characteristics of atomic structures with Zhang’s experimental data. The process took several months.
With each passing day, the excitement grew. “We were periodically checking, ‘Oh, is it still good? Still stable? The postdoc at UIUC [Juan Perilla, a PhD who was instrumental to the project] and I talked almost every other day,” says Zhang.
In the end, they found it was indeed stable. The capsid was made up of 4 million atoms—216 hexagons and 12 pentagons—consistent with Zhang’s experimental data.
At specific regions of the proteins, they found critical interactions in the capsid’s seams—details no one had ever seen before. These seams are what give the capsid the flexibility it needs to open and close at just the right time.
Further, they found that if they replaced just a few “stitches” in the seams with some small mutation, the virus tripped up. If the seam was too strong, it wouldn’t come apart when it needed to. If it was too weak, it would spring open before the Trojan horse made it into the cell. Small aberrations in the capsids would damage—or even wipe out—HIV’s infectivity.
When the embargo was lifted on the night before the Nature paper was published, a media firestorm ignited. BBC called first. Outlets from around the world followed.
This paper represents just one part of the team’s larger effort to understand and unhinge HIV. (Zhang has developed a number of new microscopy technologies for these purposes.) They’re also studying the virus’ process of maturation, as well as host-cell factors that can combat HIV. One host-cell factor the group has already published on, called TRIM5alpha, is found in many mammalian species, but the rhesus monkey version of this protein actually breaks apart the capsid. “So the capsid is really the key for a lot of drug-development targeting stuff,” says Zhang.
Now, the team is also working to develop compounds that target the newfound vulnerabilities in the capsid. We’d love to tell you more about these ongoing, yet-unpublished studies, but that would be putting the cart before the horse.
