As a med student at the Albert Einstein College of Medicine, Zachary Freyberg met a woman with postpartum psychosis. She was catatonic when she arrived at the clinic. But after a few weeks of taking antipsychotics, she came out of her stupor.
In other patients taking similar drugs, Freyberg saw horrible side effects: Some developed diabetes or gained up to 40 pounds within the first month of treatment.
“I desperately wanted to understand the biological mechanisms underlying these diseases and how the medications interacted with them so we can create better treatments,” says Freyberg, who’s now an MD/PhD assistant professor of psychiatry at the University of Pittsburgh.
Although antipsychotics have been around for decades, no one really knows how they work. The one unifying clue in these processes: dopamine is involved. But precisely how the cells in our nervous system process it—or neurotransmitters in general, for that matter—has eluded scientists.
In August 2017, Neuron published a paper from Freyberg’s team that may help crack open this mystery.
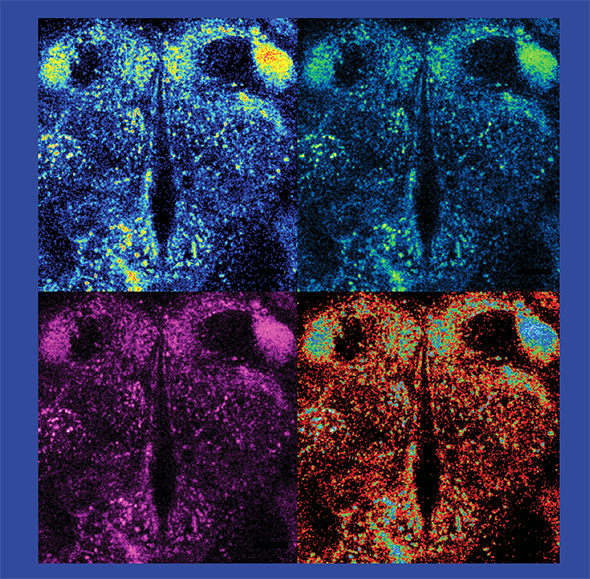
As an assistant professor at Columbia University in 2012, Freyberg started experimenting to see how neurons talked to one another. Using a new fluorescent tool called FFN206, he was able to visualize how dopamine moved around in the neurons of fruit flies: He watched as little sparks of turquoise danced, then disappeared, on a screen when he forced neurons to fire—a signal that would unload dopamine.
Pleased to see that the system worked, Freyberg’s next step was to figure out how the neuron loaded dopamine in the first place.
To move between neurons, neurotransmitters are packaged into microscopic sacs, or vesicles. Freyberg’s collaborator, David Krantz at UCLA, had a few years earlier developed a probe—a pH-sensitive, fluorescent version of a protein called VMAT, which is known to help dopamine enter vesicles. When the vesicle gets acidic, the fluorescent signal dims; as the vesicle becomes more alkaline, the signal brightens. Freyberg knew that vesicles were acidic. He decided to try his hand at this new tool, curious to see what effect, if any, pH change would have on how much dopamine was loaded into the vesicle.
After installing the pH-sensitive fluorescent VMAT probe into the brains of flies, Freyberg did a series of experiments and found that the mechanism didn’t work as he’d expected. During periods of increased activity, the pH probe dimmed and the FFN206 signal rose, suggesting that the vesicles were becoming even more acidic than when they were at rest, which caused more dopamine loading.
“At first, I thought it was a mistake, an artifact of the imaging. But it kept repeating,” he says.
Freyberg then reasoned that if the vesicles were becoming more acidic, there must be a negative charge in counterbalance, controlling how dopamine was loaded. And on further study, he found that this was exactly the case. The source of the negative charge, as it turned out, was glutamate.
This seemed counterintuitive, because glutamate is also a neurotransmitter. Previously, researchers thought neurons would release only one neurotransmitter at a time to an adjacent cell. Freyberg’s studies suggest that, actually, neurons can multitask and release two neurotransmitters at once. Further, the team found that neurons tailor the amount of neurotransmitter they release based on how strong the incoming message is; the harder a neuron fires, the more acidic the vesicles get, which helps more dopamine get in.
Freyberg has validated the fruit-fly findings in mice. In the near term, he’s looking to see if the concomitant release of dopamine and glutamate is relevant in neurological diseases. “That’s the million dollar question right now,” he says.
A Day to Connect Those Who Teach
BY ELAINE VITONE

Pitt Med has long had a rep for being the sort of place where scientists crosstalk easily outside of the departmental crowd they might normally roll with. All that confabbing has strengthened scholarship, community, and collaborations in enviable ways, says Dena Hofkosh.
Well, for researchers, that is. For educators, not so much.
“I realized that while there were many interesting and innovative educational programs, [medical educators] didn’t have a way of talking to each other across departments. So educational scholarship wasn’t so easy to see.”
In 2014, when Hofkosh, director of Pitt’s pediatric residency, added associate dean of faculty affairs at the School of Medicine to her title, she set to work creating a way to bring educators together from across the health sciences to showcase successes, share insights, and pool resources. The result: an annual event called Med Ed Day, sponsored by Pitt Med’s Academy of Master Educators, Office of Faculty Affairs, and Office of the Vice Dean, as well as the Office of Academic Career Development, Health Sciences. The Academy of Master Educators is now planning Med Ed Day’s third iteration for September 2018.
Last year’s event drew faculty, staff, students, residents, and fellows from across the health sciences. Ohio State University’s Quinn Capers IV gave the keynote address on mitigating unconscious racial bias in the med school admissions process. (One gem of a takeaway: Training the Ohio State admissions team regarding bias didn’t increase the school’s acceptance rate of students from underrepresented groups, but it did increase the rate at which those students enrolled!)
The keynote was followed by a series of TED-style talks full of Pitt Med-ucators’ insights: teaching budding clinicians how to share the decision-making process with their patients; helping surgical residents think on their feet in the O.R.; using group video review of classroom encounters to help faculty members learn from one another; showing trainees how to spot the kinds of cognitive errors that can lead to misdiagnoses; and using students’ own genetic data to teach precision medicine.
Some presentations described brand new curricular innovations coming down the pike; others were more established educational research findings, with supporting data on a curriculum’s impact on knowledge, skills, and attitudes. The latter represented an exciting new shift in the field, says Ankur Doshi (MD ’00), associate professor of emergency medicine at Pitt who was on the Med Ed planning committee. Just as good medical practice is rooted in evidence, so is good teaching. “And we’re really moving toward that in medical education,” he says. Historically, grant support for medical education scholarship has been hard to come by—there are no National Institutes of Health grants devoted to it. But an increasing number of teachers are scaring up funding in creative ways; that’s a popular topic of discussion for Med Ed goers, he adds.
At the poster session last fall, crammed into the Biomedical Science Tower lobby, various Med Ed scholars networked around topics ranging from interprofessionalism to simulation-based instruction. In the thick of all that elbow-rubbing, a problem emerged, says Hofkosh: They needed a bigger room.
Between a clot and a hard place
When Platelets Go Bad
BY ALLA KATSNELSON
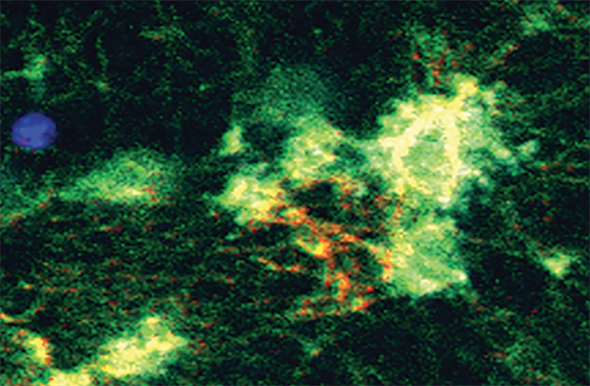
Platelets—the body’s internal Band-Aids—are mighty shape-shifters. These cell fragments, which make up just 1–2 percent of your blood, roam your circulatory system in the form of tiny convex discs. When they sense damage to blood vessels, they home in on the injured site, attach to the vessel walls, and hit the proverbial Superman phone booth, metamorphosing into sticky, tentacled blobs. Through a slew of emitted and received molecular signals, these now-activated platelets entangle themselves with the threads of fibrin protein to stanch blood flow.
Sometimes, though, this crucial system malfunctions. Take trauma, for instance—the leading cause of death for people younger than 45. In about one in four trauma patients, the body’s ability to stop bleeding breaks down. Some 40 percent of trauma patients who die do so by hemorrhaging.
Ironically, those who survive acute injury must contend with an opposing concern: Activated platelets often stay sticky longer than they seemingly should, dramatically raising the risk of blood clots.
“One of the most incredibly frustrating things as a trauma surgeon is to resuscitate someone from the brink of death and get them through that vicious cycle of not being able to stop bleeding, and then have them die three days later from a blood clot,” says Matthew D. Neal (MD ’06, Res ’14, Fel ’15), assistant professor of surgery and critical care medicine at the University of Pittsburgh.
Neal believes that the swing between these two extremes is caused by injury-induced signaling between platelets and the innate immune system, which regulates inflammation. This fall, his laboratory received a $1.8 million grant from the National Institute of General Medical Sciences. He was selected for a Maximizing Investigators’ Research Award to elucidate such signaling and to tackle the major clinical problems it causes.
Neal and his colleagues aim to build on findings they reported in the Journal of Clinical Investigation in 2015; those showed that activated platelets express a molecule that opens the inflammatory floodgates. In turn, the same molecule keeps platelets in their sticky, activated form.
Researchers have long known that trauma kicks off inflammation, but that study provided some of the clearest evidence that platelets do more than just help stop bleeding.
“I think the involvement of platelets in the overall inflammatory cascade was really very surprising,” Neal says.
Modern trauma care and the evolution of the innate immune system are out of balance. Surgeons today may be able to save us from dire circumstances that evolution didn’t account for (multiple gunshots, car crashes . . .). But that medical magic is not always perfectly suited to the immune response: platelets can remain excessively activated, Neal explains, “doing their day job in places where they shouldn’t.”
Neal and his colleagues want to more clearly understand the innate immune signaling pathways that affect platelets and how this signaling causes blood clots to form. In a study forthcoming in Scientific Reports, the researchers will show that the interaction between platelets and immune cells called neutrophils revs up these pathways—and, along with them, clot formation.
The team is also studying how platelets change after trauma. They hope to identify characteristics that define the subset of platelets that are clot-forming troublemakers.
Such in-depth knowledge of platelets could yield many medical benefits, Neal says. In October, he received an additional grant from the Department of Defense in collaboration with bioengineer Anirban Sen Gupta at Case Western Reserve University to create synthetic platelets that could be used on the battlefield or in remote settings. The substance they developed appears to limit blood loss in mouse and pig models, according to two recently published studies.
The grant also funds a nanotech project to create a molecular drone that can target activated platelets and deliver a drug that dials back their immune signaling and thus their clotting potential. That way, Neal says, “we can allow the platelet to do its day job, but attenuate the negative consequences.”
Platelet image republished with permission of AMERICAN SOCIETY FOR CLINICAL INVESTIGATION, from Vogel S, Bodenstein R, Chen Q, et al. “Platelet-derived HMGB1 is a critical mediator of thrombosis.” Journal of Clinical Investigation. 2015;125(12):4638-4654. doi:10.1172/JCI81660