
A couple of years ago, a group of Pitt researchers decided the humble eyedrop was due for an upgrade. Eyedrops are messy and sometimes prone to interact with other medications—“a terrible way to get drugs into your body,” Morgan Fedorchak, a PhD assistant professor of ophthalmology, says.
So the group got creative and invented an alternative: SoliDrop, a slow-release eye gel.
Then they got stuck.
Coming up with a neat medical technology in the academic setting is one thing. Commercializing it is quite another. You need a striking study, a snazzy prototype, an airtight business plan—all of which require money most grants don’t cover and skills most professors haven’t learned. This stage between innovation and commercialization is often referred to as the valley of death.
But the SoliDrop researchers are optimistic. That multidisciplinary team includes Fedorchak, who helped develop the gel alongside Steven Little, a PhD and Pitt’s chemical engineering chair (among other titles), Ian Conner, an MD/PhD and assistant professor of ophthalmology, and Joel Schuman, an MD and former chair of ophthalmology. In hopes of seeing the gel in patients someday, Fedorchak and Conner are taking a crash course in how to commercialize the gel, and they’ve gotten sufficient funds to put the invention in front of external investors—thanks to the Coulter Translational Research Partners II Program at the University of Pittsburgh—or Coulter @ Pitt.
The program helps researchers with new medical technology ideas attract investment. Each year, five $100,000 Coulter grants go to Pitt clinician-engineer teams that have identified an unmet clinical need and come up with a feasible, patentable solution.
But “last-mile money,” in the words of Coulter project manager Suneera Bhatia, is not enough. The teams also have to learn how to get an idea ready for licensure or a new company. In the jargon of commerce, this process is called de-risking, and Coulter @ Pitt teaches researchers how to do it. When it’s time to present to potential investors, the researchers are boardroom ready.
Previous awardees, Fedorchak recalls, told her to be prepared—“that [Coulter was] going to take us outside of our comfort zone as scientists, as purely academicians. I thought I was ready for that, but it definitely was a challenge,” she says. “I learned so much through the process.” If the name Coulter sounds familiar, you may have heard of the inventor Wallace Coulter. He patented an automated blood-cell counting device in 1953, later founding the company that became Beckman Coulter.
Coulter @ Pitt funds come mostly from a five-year, $667,000 per year grant made by the Wallace H. Coulter Foundation to the Swanson School of Engineering’s bioengineering department in 2011. Only 16 U.S. universities have received these grants.
At Pitt, additional institutional contributions—from the Swanson School of Engineering, Schools of the Health Sciences, Office of the Vice Provost for Research, and Innovation Institute— bring Coulter @ Pitt’s annual budget to $1.2 million. Each year, five innovator teams chosen by the Center for Medical Innovation are awarded $200,000 to seed their projects.
“Whatever that last barrier is, or last set of barriers, [Coulter] will fund that,” says David Brienza, a PhD and professor of rehabilitation science and technology and of bioengineering, who is another Coulter grant awardee. Potential barriers might include clinical studies or marketing research.
Figuring out those needs is a high priority—and happens before any project is funded. Each spring, after passing an early selection round, seven to 10 candidate clinician-engineer teams enroll in a semester-long course called From Benchtop to Bedside. Nicknamed B2B, the course is taught by serial entrepreneur Babs Carryer, director of education and outreach at Pitt’s Innovation Institute, along with noted guest speakers from industry.
In B2B, doctors learn to do market research. Engineers learn when to call a clunky prototype good enough. And everyone learns the language of business. The teams meet with students from Pitt’s law and business schools to put together business plans. They study regulatory requirements, intellectual property rights, and market-research techniques. They think deeply about who their customers are. In the process, they add rigor and clarity to their proposals and ultimately develop a polished pitch for investors.
After completing B2B, five teams are chosen to receive grant money. They’re each assigned to a project manager who helps them set and navigate milestones. Each team is co-led by a clinician and a scientist/engineer.
Teams get plenty of advice and mentoring, beginning with an oversight committee, a kind of “board of directors” who come from clinical practice, industry, academia, venture capital, regulatory affairs, and health care economics. Additionally, some 80 advisors serve as Coulter @ Pitt mentors, including Sanjeev Shroff, a PhD who is principal investigator of the program, as well as chair of the Department of Bioengineering; co-PI Stephen Badylak, a DVM, MD/PhD, and deputy director of the McGowan Institute for Regenerative Medicine; and co-PI Marc Malandro, a PhD and the Innovation Institute’s founding director. Other advisors include angel investors, medtech business leaders, and people who know how to navigate regulatory hurdles at the Food and Drug Administration and its equivalents abroad.
Coulter @ Pitt collaborates with the med school’s Clinical and Translational Science Institute, which works with researchers at an earlier stage in the innovation process. At a later stage, the Innovation Institute serves as “our pipeline to the market,” as Coulter @ Pitt director Max Fedor puts it, helping license Coulter-packaged technologies to an existing company or start a new one.
Coulter defines a success as at least $500,000 in professional investment. By that measure, Pitt’s program has had several.
Two new companies have formed around Coulter @ Pitt innovations. One, NanoVision Diagnostics, offers an early cancer detection technology developed by Yang Liu, a PhD associate professor in the departments of medicine and bioengineering. Leading the clinical side of the project are co-PIs Randall E. Brand, an MD professor of medicine, and Rohit Bhargava, an MD associate professor of pathology.
The other company, Formabone, is commercializing a resorbable bone putty developed by bioengineering professor Prashant Kumta, a PhD (who has several other engineering and dental school appointments), and periodontics chair Charles Sfeir, a DDS and PhD who is also director of the Center for Craniofacial Regeneration. Sfeir is also working on another Coulter project.
Two Coulter software products—InterACTION, which assists physical therapists during joint rehabilitation, and PIVOT, which helps diagnose ligament tears in the knee—have been optioned by Impellia, a company founded by retired Pittsburgh Steelers quarterback Charlie Batch. Several other projects, according to Fedor, are “ripe” and ready to move into licenses or startups soon.
The whole process can be humbling, with the professor becoming a student once again. But Fedorchak says it’s rewarding: “This is what every scientist hopes for, right? Not to be hunched over a bench their entire career and never have anything get outside the laboratory,” she says. “I see it as a very welcome kind of problem and work to do.”
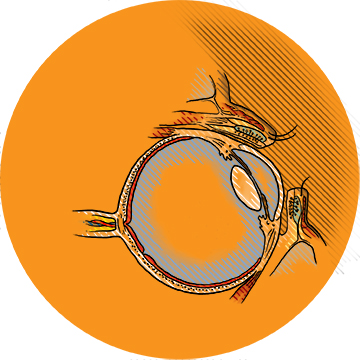
Make Eyedrops Gel
Glaucoma patients are critically dependent on eyedrops to help control the sight-threatening rise of pressure within the eye. But eyedrops may be so poorly absorbed that they have to be applied several times a day in high doses—a regimen that can be almost impossible to adhere to and can cause unwanted side effects.
“Eyedrops are very inefficient,” says assistant professor of ophthalmology Morgan Fedorchak (PhD ’11). “There are all these permeability barriers designed by nature to keep things out of your eye.”
SoliDrop, whose inventor team includes Fedorchak and assistant professor of ophthalmology Ian Conner, is administered like an eyedrop, but then at body temperature turns into a gel. It settles in the pocket under the lower eyelid and slowly releases the drug over the course of a month. In animals, Fedorchak says, the gel stays put and has great therapeutic effect. (Fedorchak is also an assistant professor of chemical and petroleum engineering; Conner, who holds a secondary appointment as assistant professor of bioengineering, directs the UPMC Eye Center in Bethel Park.)
Coulter money has allowed the SoliDrop team to start gathering safety data to bring to the FDA for an Investigational New Drug (IND) application. At the same time, the team is looking into licensing strategies and partnerships to fund an IND clinical trial.
Fedorchak says she learned so much in the B2B course that being chosen to receive funding was “icing on the cake.” And Coulter’s flexibility regarding how funds are spent, she says, has given them great freedom, allowing her and Conner to hire business consultants, look into manufacturing methods, and do toxicity testing.
Lesson learned: Be nimble. When Fedorchak and Conner realized they needed to change their original milestones—a change that would affect tens of thousands of dollars’ worth of their Coulter funds—Fedorchak gingerly approached the team’s project manager, Shubhangi Lal, with a revised plan of action.
“She didn’t even blink. She was like, ‘Okay, let’s do it,’” Fedorchak recalls. “The buzzword is ‘pivot.’ We’ve pivoted rather dramatically. It’s great.”

Keep It Cool
Lying flat in bed for days or weeks can lead to pressure ulcers in critically ill and injured patients. As the skin over the sacrum—the large bone at the bottom of the spine—suffers unrelenting pressure and friction, it can break down, causing wounds that sometimes require surgical removal of large areas of skin and muscle. These ulcers can be difficult to prevent and harder to reverse. In the United States, their burden is estimated as high as $11.6 billion annually.
Enter PRO-TECT, an air mattress with a cooling gel inside. As the body exerts downward pressure at the sacrum, the cooling elements conduct heat away from that area of the body. This allows the tissue to withstand the pressure and shear force, without causing or worsening an ulcer—or lowering the patient’s overall body temperature.
PRO-TECT is the brainchild of David Brienza, a PhD and a professor of rehabilitation science and technology and of bioengineering, and trauma surgeon Alan Murdock, an MD; it grew from Brienza’s research on wheelchair cushions. The team is now engaged in a clinical trial.
Lesson learned: Ugly can be beautiful. An engineer’s perfectionism can be the enemy of good business development—at least at first.
True to form, Brienza began intent on a polished product. But potential licensees told the team it might need to redevelop the product anyway. The Pitt group learned to focus on providing evidence that the mattress could be effective, developing a functioning prototype for testing purposes instead.
“We wanted to perfect the product,” says Brienza. “Then that all went out the window. We said, ‘Let’s just make something that works, even if it’s ugly.’”
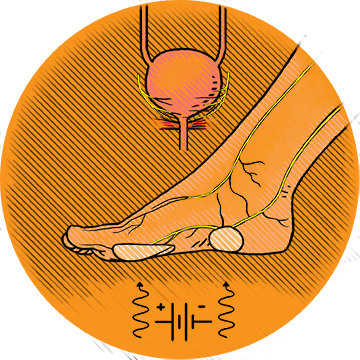
Soleful Stimulation
Neuromodulation is a mysterious but effective therapy for a number of disorders. Somehow, if you electrically stimulate a peripheral nerve, that stimulation can send signals along the spinal cord and cause changes in brain neurotransmitters; and those neurotransmitters can affect a seemingly unrelated part of the body, including the muscles controlling the bladder. One FDA-approved overactive bladder (OAB) neuromodulation device on the market requires weekly in-office neuromodulation sessions delivered via a needle inserted behind the ankle.
The FootStim team takes a different approach. Instead of needles, FootStim uses stickers on the sole of the foot to deliver the electrical pulses to branches of the tibial nerve. This noninvasive device could be used by patients at home. It would also be a cheaper alternative.
In a study of eight healthy human volunteers, a single 90-minute stimulation session with FootStim led to a temporary 200 cubic centimeter increase in bladder capacity, with no side effects. A second study of 19 women with a form of urinary incontinence found that 16 responded to a nightly three-hour session with FootStim, with statistically significant reductions in symptoms.
More studies need to be done, says assistant professor of urology Christopher Chermansky, an MD. But if the technology is effective, it could hit the market within three to five years. Already, FootStim has caught the eye of a company in California, and the team hopes to license their product next year.
Chermansky’s co-PI, associate professor of urology Changfeng Tai, a PhD, says Coulter money is very different from a National Institutes of Health grant. “Less than half is about the science,” he says. “More than half is about development, business, venture investment, all those things. They asked us to develop a business plan. I never [had to write] that before,” he adds, laughing.
Lesson learned: Business is, in large part, who you know. The biggest advantage to the Coulter experience, Tai says, was the links it provided to business people. You can’t move forward without the right alliances.“Without Coulter, we [would] never meet those people,” he says.
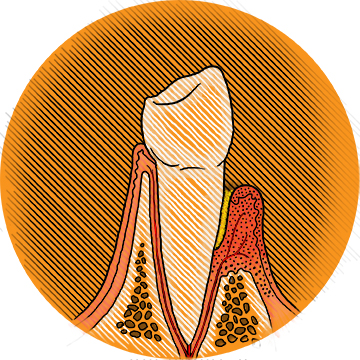
Immune to Gum Disease
Getting your teeth professionally cleaned to reduce their bacterial load around the gumline has long been a mainstay in preventing and treating periodontitis (gum disease). But periodontics professor and chair Charles Sfeir, a DDS and PhD, and Steven Little, a PhD, the William Kepler Whiteford Professor and Chair of Chemical Engineering, and professor of immunology, think there’s a better way.
In periodontitis, the immune system is actually part of the problem. Though bacteria trigger the disease, it’s when immune cells overreact to those bacteria that the accompanying bone destruction takes place. By contrast, when it comes to tumors, the danger is when the immune system fails to act. That’s the clue Sfeir and Little were looking for.
Tumors ought to be highly inflammatory, alerting immune cells to destroy them. Yet they can survive, in part, by deceiving the immune system. Tumors exude a chemical signal called CCL22. That signal binds to receptors on regulatory immune cells, and those regulatory cells then alter the surrounding population of immune cells in favor of nondestructiveness. In effect, the tumors broadcast an “all is well” message.
Sfeir and Little devised a way to package CCL22 into degradable polymers that are deposited in powdered form beneath the gumline, where they stick in place and allow for sustained release of CCL22. As it does with tumors, the chemical signal promotes an anti-inflammatory environment in the immediate surroundings.
They’ve gotten a similar approach to work in animal models of dry eye disease— an inflammatory breakdown of the tear ducts—and even in limb transplants.
“We have rats that are walking around for over 200 days with another rat’s leg, and they’re on no systemic immunosuppression with these treatments,” Little says.
The team is now looking for further funds with which to produce a patient-grade version of CCL22 and begin a safety study. “Coulter money is kind of keeping us alive,” Little says.
Lesson learned: Grants aren't just for science anymore. The Coulter @ Pitt’s lessons weren’t entirely new to Little and Sfeir, who say they already had a good grasp of the commercialization process. But Sfeir has observed that Coulter changes the way researchers think about a grant—“You’re not just doing it to publish papers,” he says. Instead, Coulter mentors advise researchers to allocate funds for, say, consultants who can prepare pitches and do market research. “There’s a bit more oversight than you would see in a regular grant, and that’s very intentional on Coulter’s part,” Sfeir says.
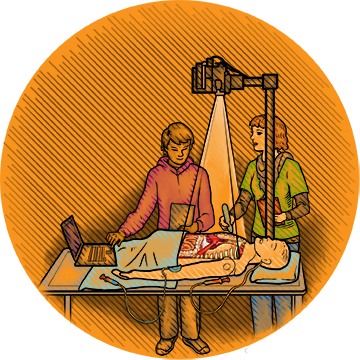
Walk Up Learning
Joseph Samosky, a PhD assistant professor of bioengineering and director of Pitt’s Simulation and Medical Technology Research and Development Center, is passionate about building a better medical simulator. His connection to the subject is personal: Both his parents suffered from medical errors, and better training might have prevented those mistakes. Plenty of evidence shows that simulator training translates to better performance among providers, he says.
That said, modern-day simulators can be cumbersome. They’re bulky and nonversatile, and they require dedicated infrastructure and costly technician time. Moreover, they don’t teach students anything on their own.
So Samosky and Pitt’s John O’Donnell, a DrPH professor and chair of nurse anesthesia, are developing BodyExplorer, an expandable, customizable training system that will offer multiple plug-and-play training options—such as breathing-tube placement or IV medication administration techniques—much the way a smartphone runs apps. BodyExplorer is intended to be so user-friendly that a student could walk up, turn it on, and start learning any of a wide array of tasks, complete with plenty of feedback on her performance.
Investors have noticed. Samosky’s team is in discussions with a party regarding licensing the product, and a new company could be developed as early as next year.
Lesson learned: Consider all the stakeholders. As an engineer, Samosky figured at first, naturally enough, that the person he needed to bear in mind as he designed BodyExplorer would be the health science student who’d be training on it.
“But here’s the thing: The student is not the person buying the system,” Samosky says. It’s essential to consider the perspective of a med school or hospital administrator who’s wondering if she needs to build a simulation center or can cut costs by using a turnkey, on-demand learning system like BodyExplorer. Coulter’s training helped him refine that vision.
No Longer Torn
Tears in the anterior cruciate ligament (ACL) are among the most common knee injuries—200,000 such injuries befall Americans each year. The ACL typically heals so poorly that surgical reconstruction—replacing the injured ligament with a graft—has been a go-to treatment for decades.
Yet reconstruction is less than ideal, says bioengineering professor Savio L-Y. Woo, a PhD, DSc, and DEng who is also on the core faculty at the McGowan Institute for Regenerative Medicine. Postoperative complications like osteoarthritis are common, and papers on surgical techniques for ACL reconstruction are being published all the time.
“When you have a procedure that has that many techniques,” Woo says, “obviously something is not working right.”
Woo, scientific PI, and his partnering clinical PI, associate professor of bioengineering Patrick McMahon, an MD, invented an alternative device, one that could heal the ACL and eliminate the need to replace it with a graft. Called LigaMend, it consists of FDA-approved extracellular matrix bioscaffolds and a collarlike resorbable magnesium ring. The ring loads the ligament where it attaches to the femur, preventing that attachment from weakening from lack of use during the long healing period. The bioscaffolding accelerates healing after surgical reconnection of the injured ligament. Studies in animals have shown promising results at 12 weeks, and the team is looking to carry that through to 26 weeks.
“If the attachment site is maintained and [does] not deteriorate,” Woo says, “then we’ve really got ourselves a brand-new approach.”
Lesson learned: Time it. “As academics, we don’t work on a timeline,” Woo says. “One question leads to another question, because we’re not product-oriented, at least me. [But] the world is changing, and I need to adapt.”
For more info on Coulter @ Pitt, which accepts applications each fall, contact Max Fedor at maf210@pitt.edu.
